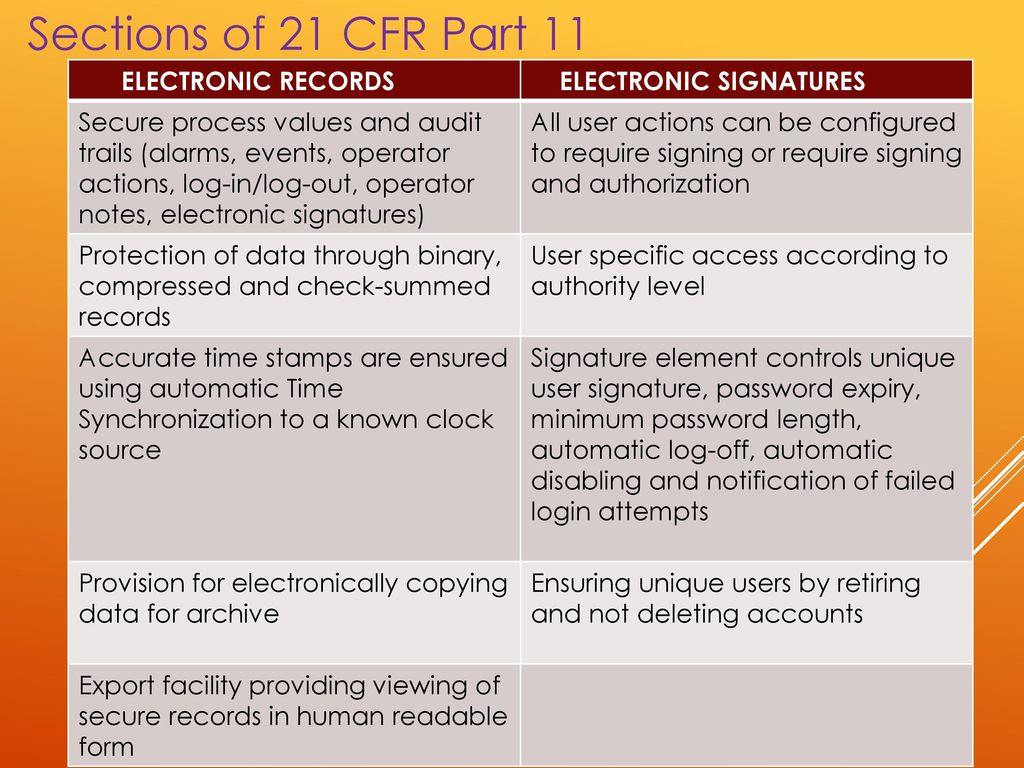
What Is So Fascinating About 21 Cfr Part 11 Compliance for Excel Spreadsheets?
Sufficient space ought to be provided for such entries. A critical element of effective high quality management is timely accessibility to accurate and total high quality data. The materials might be duplicated for anybody attending. Google Forms also makes it simple to collaborate on eCRF design with different researchers.
Top Guide of 21 Cfr Part 11 Compliance for Excel Spreadsheets
Spreadsheets are a rather strong and useful tool in the GxP environment especially when coupled with the simple fact that it’s simple to construct spreadsheet applications without a lot of training. This makes spreadsheets an extremely strong and useful tool in the GxP environment especially when coupled with the simple fact that it is not hard to build spreadsheet applications without a lot of training. When a document was revised, systems ought to be operated to stop inadvertent use of superseded documents. This is a robust and easy to execute document, one that is going to lead you through the procedure and deliver a result that may be used as the foundation for your validation activities. Reproduced documents ought to be clear and legible. Understand what validation documentation is necessary. These kinds of Part 11 validation are the ones to focus on.
What 21 Cfr Part 11 Compliance for Excel Spreadsheets Is – and What it Is Not
Webinar might be cancelled as a result of absence of enrolment or unavoidable elements. A third trigger event to contemplate is if your organization undertakes a new small business partner or a new supplier which will be creating regulated data for your benefit, storing regulated data for your benefit, or otherwise manipulating and processing regulated data for your benefit. There are not any prizes for guessing what this file format is it actually is a spreadsheet! On the other hand, the FDA expects that spreadsheets be compliant and absence of compliance can lead to a warning letter. However, it expects that spreadsheets are compliant and lack of compliance can result in a warning letter. This will aid FDA to redact the proper information whilst providing you some level of assurance they understand and acknowledge the circumstance. The FDA isn’t blind to reality.
In some instances it is a hands-on mentoring procedure. Even otherwise, these applications are simple enough to master by yourself. These tools also don’t provide guidance on eCRF design. This simple-to-use software utilizes a web-browser interface and so can be employed with a wide variety of devices. It is necessary for software vendors, auditors, and excellent staff involved with GxP applications.
To maintain compliance, system administrators should have a system which provides the capacity to delineate user permissions for every single document vault in the computer system. Employees have to be given the chance to finish a particular quantity of training every year and the time spent in training has to be accounted for. Companies operating in several countries should consider regulations when picking an LMS and be certain that system supports the applicable regulations. And it isn’t just because it’s very good for business either. Companies beyond Germany can be less stringent since they aren’t required to obey German regulations. Organizations are permitted to retain their data in a totally digital format.
No commitment to proceed to purchase or create the spreadsheet needs to be taken until the DQ was executed. Also there’s no guarantee you will see the information specific to your study in the long run. Refunds won’t be given to participants who don’t appear for the webinar.
The capacity for a signer to repudiate an approval has to be minimized. But these have a tendency to be complicated. They’re described here to be able to emphasis their relationships and their fundamental value to the manufacturing and control of medicinal products. You shouldn’t be wasting your time this manner. We guarantee a reply to your query within a day on weekdays no matter your time zone. Every so frequently, it’s better to recalibrate by looking at frequently asked questions as soon as it comes to FDA inspections. It will talk about the frequent compliance problems with spreadsheets and the way you can stay away from them.
Therefore, during inspections, it’s technically collecting evidence. With this internet program, you may set up your study in a few days. In others it’s much like an evaluation. Implementation of an electronic system with an established history of performance and validation can drastically decrease the time and money a business devotes to its general validation efforts. The growth of Excel template is often achieved by specific users.
No exceptional understanding of the CyberLAB application is required for the end user. With regulations tightening around the world, non-compliance with, as an example, Good Clinical Practice (GCP) is an issue for all researchers involved with clinical trials. When training occurs outside of normal work hours employers could be asked to compensate employees for that moment. SEE ALSO : 2017 tax planning spreadsheet
Sample for 21 Cfr Part 11 Compliance For Excel Spreadsheets